My burning bush, in the fall of 2009
I have a burning bush. Not the biblical kind, mind you. Euonymus alatus. The kind of bush that looks like a normal green bush for most of the summer, but then turns into a wild thing in the fall. (Discretion tells me this would be a good time to not mention my wife.)
Fall is coming. And my euonymus is doing it's magical chromatic transmogrification.
My burning bush, just starting to warm up
What better time to get out my spectrophotometer? Here are the leaves I measured - several spots on each of the lovely leaves.
Selected leaves from the bush
First step, I show the color in CIELAB values. This is an a*b* plot.
Color values (a*b*) of spots on the leaves
I look at this marvelous plot and it just makes me wonder "why"? What changes in the leaf to make this marvelous color change? I start by look at the reflectance spectra. This makes a cute plot, but I'm not sure I can gather much from it, other than verifying that there was a distinct color change. How about I look at the spectra?
Corresponding reflectance spectra
The reflectance spectra plot is a bit more interesting. From this, I can see three things. First, at the blue end of the spectrum, something is doing a whole lot of absorbing. Why do I say that? Because the reflectance is so low. Someone is stealing most of the blue photons! This is true of all the measurements, so I am going to say this is one pigment.
Second, I see that in the green leaves, there is something that absorbs a lot of light at the red end. It doesn't absorb as much in the green region. Maybe it absorbs in the blue as well, but I can't tell. I'm going to call this a second pigment, and tentatively give it the clever name "green".
Third, in the red leaves, there is something that absorbs a whole bunch of green, but not much red. I'm going to tentatively name this proposed pigment "red". Again, a clever name choice on my part.
Now... I am going to make a bold assertion here. I think that as the leaf changes from green to red, the pigment "green" leaves the leaf and the pigment that I affectionately named "red" jumps in to replace it. Why do I say that? Note that the reflectance of the red leaves is pretty gosh darn high at 700 nm, but the really green leaves absorb a great deal of the light at 700 nm. Clearly there isn't much left of whatever absorbs the 700 nm light.
Despite not being a chemist, I am going to take the analysis one or two steps beyond my skill level. The reflectance spectra show a view that is useful for someone interested in the color of the leaves, since it looks at what the eye sees. But the reflectance view is not so useful to someone analyzing what the leaves is doing. For that, one would want to look at the absorbance spectra, AKA density spectra.
Absorbance spectra of leaves
There is no real magic math here. All I did was ask Excel to take the negative of the logarithm of each reflectance value. And what it gives me is a plot that is a gauge of the absorbed light. So, note that the "red" and "green" have been inverted like an euglena haplessly swimming on a microscope slide, oblivious to the fact that it is being watched.
Another nice feature of the absorbance plot is that it is kinda sorta a little bit additive. In other words, when you cut the concentration of a pigment in half, you kinda sort divide the absorbance in half. And even cooler, when you add two pigments together, you kinda sorta add the two absorbance spectra together. Need a bit more information on that? Check out my post on Beer's law.
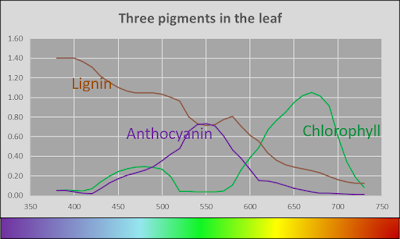
With that knowledge in mind, I took a wild stab at what the spectra of three pigments might be, and went further out on a limb to guess what the actual pigments might be.
Plausible absorbance spectra of pigments in the leaves
Lignin is the brown pigment that makes grocery bag brown. Lignin is the bane of paper making. Paper mills go to great lengths to ask lignin to leave the pulp, cuz people like their paper to be white. Did I mention that lignin is brown? I am pretty sure that lignin is in most all leaves, and this would account for the fact that all the leaves reflected very little light at the blue end of the spectrum. The lignin purloined the cerulean photons.
Chlorophyll is a pigment that everyone has heard of. This is what makes leaves green (by absorbing red and blue light) and is the active ingredient in photosynthesis (by using the energy absorbed of the red and blue light). Everything that I remember from elementary school science would be a lie if I didn't find chlorophyll in the leaves. I have proposed that chlorophyll absorbs a bunch of light at the blue end, but not a great deal. But this is just my guess.
And then we come to the red pigment. I am going to take a wild guess and say that the pigment is anthocyananin, mostly cuz I like the sound of it. And it makes it sound like the red leaves of a burning bush might provide you with all the anti-oxidants that keep you from getting into oxidants on the highway. Again, just a guess on my part.
The assignment of colors in the absorbance plot above is likely a bit confusing. I found myself getting confused. This is not an unusual condition for me. So, let's go back to the color scientist view, and show the reflectance plots of the plausible spectra of the three pigments.
Plausible absorbance spectra of pigments in the leaves
So. There you have it. Another discourse where I take a thing of beauty, the burning bush, and turn it into a lousy science lesson.







nice post! here's a link that may interest you
ReplyDeletehttp://opticleaf.ipgp.fr/index.php?page=prospect
It's an optical model called PROSPECT where you can compute a leaf reflectance spectra using the leaf's characteristics. Have fun!
Excellent, Theo! I was hoping that I would get a comment from someone who actually knows something about leaves!!
ReplyDeleteWell actually my knowledge about them leaves a lot to be desired!
ReplyDelete