I said in a previous blog post that I wanted to talk about fluorescent bulbs. I do. Really. And I will... in this very blog post. But before we get to that dessert, we need to eat our peas and carrots. Let's talk about fluorescence.
The soon-to-be-famous sunburn analogy
I am of Northern European stock. I sunburn easily. Naturally, I wound up in a place where the Sun doesn't shine. Milwaukee. On those rare occasions when the Sun does shine, I absorb ultraviolet light. Later, my skin emits red light.
Lobster, anyone?
That's fluorescence.
Well, not really. I do absorb UV, and my skin does turn red. But that red is a reflective red, rather than a emissive red. My skin doesn't actually give off light. Factoid: sunburnt skin is red due to the increased concentration of hemoglobin at the surface. Hemoglobin absorbs bucketloads of light in the OYGBIV part of the spectrum, and reflects some at the R end. The reflected light is thus comprised chiefly of red light so skin looks red when we burn. (Interested in more about the color of human skin?)
Just in case you were wondering, my normally pasty-white Anglo-Swedish skin matches 2R04 in the Pantone Skintone guide.
If I recall correctly, though, I was talking about fluorescence. My explanation about sunburn shares a lot of the features of fluorescence. Light is absorbed at one wavelength, and is emitted at another wavelength. It is always emitted at a wavelength with less energy, which is to say, at the more relaxed higher wavelengths. For some molecules, the absorbed light is in the UV, and the emitted light could be at the red region of the spectrum.
My understanding of the fizzicks involved
This will thankfully be a short section. I dunno nothin' about the fizzicks behind fluorescence. I mean, a molecule absorbs a photon, and that photon "kicks it up into a higher energy state". I have no clue what that means. I just know that I don't want to be around when my wife gets kicked up into a higher energy state.
Later, the excited molecule gives up that energy, but not all at once. For some reason, it only gives it up a parcel at a time. Hence each fluorescent emission is at a lower energy (higher wavelength) than the excitation.
Happy little benzine molecule
Later, the excited molecule gives up that energy, but not all at once. For some reason, it only gives it up a parcel at a time. Hence each fluorescent emission is at a lower energy (higher wavelength) than the excitation.
Note that I said molecule, and not atom. In the last post, kicking an atom up into a higher energy state was all about the orbits of electrons. Now it's about molecules. Surely that's a clue about what is happening when something fluorescences. But I am pretty ignorant when it comes to all that chemistry stuff. I'm the guy who once looked for a quantum mechanic to fix my compact car.
But in the spirit of pretending I know something...
If I really understood any of this stuff, I would explain that
in this diagram from Kurt Nassau's book,
the wavy lines represent fluorescence
in this diagram from Kurt Nassau's book,
the wavy lines represent fluorescence
But in the spirit of pretending I know something...
There is a closely linked phenomenon called phosphoresence. Actually, it's the same phenomenon with a different name. Light is absorbed and is later emitted at a higher wavelength. The only difference is in how much later the emission happens. If it happens on a time scale where we don't notice (like nanoseconds or milliseconds), it's called fluorescence. If the delay happens on a time scale that we notice, for example if the fluorescent emission continues for seconds or hours after the excitation goes away, then we call it phosphorescence.
The distinction between fluorescence and phosphorescence is thus strictly anthropocentric. Just like the distinction between electromagnetic radiation and light (described in a previous blog post), the distinction is along a continuum and is not based on anything physical other than our meager, pitiful senses.
Examples of phosphorescence
Back in the olden days, engineers made a lot of use of phosphorescence. Cathode ray tubes (CRTs) in electron microscopes and in radar systems had long-persistence phosphors so that the image stayed latent on the tube long enough for us to notice. Quick show of hands... how many in the audience have used one of these devices?
Ok... let's try to open this up a bit. Show of hands again. How many in the audience have been to a historical museum (or my basement, same thing) and saw a TV that was two feett deep and weighed more than a pregnant and cross-eyed mule? That, my friend, was a cathode ray tube display, with a phosphor on the inside of the display end of the tube. Then again... maybe that was more accurately called a fluorescor, since we definitely didn't want it to persist for more than a 30th of a second.
(By the way, a scanning electron microscope is nearly identical in structure to the cathode ray tube in a television set. In fact, the cathode ray tube display was invented along side the scanning electron microscope. Someday I will blog on that topic.)
Certain lichens and mushrooms will glow in the dark long after the sun has gone down. Perhaps there is an evolutionary advantage to being seen as a part of the night life of the forest? I dunno. When it comes down to it, being a visible part of the night life has never given me much of an evolutionary advantage. It usually kicks my wife up into a higher energy state.
Some minerals fluoresce like an Anglo-Swedish color scientist with sunburn. Party-loving minerals like fluorite come to mind. I wonder where it got that cool name? Come to think of it, where did phosphorous get it's cool name?
You can also see phosphorescence if you look at a fluorescent bulb in the dark, just after it has been turned off. Here we see the vague distinction between fluorescent and phosphorescent. Some of the stuff inside the tube is fluorescing, and some of it is phosphorescing. But more on that when I finally get around to discussing fluorescent bulbs.
But by far the most useful application is the little rubber duckie that was sitting on my wife's desk, at least until I absconded with it for a photo shoot. The rubber duckie is impreganted with some phosphor with excitation in the violet to blue part of the rainbow, and emission in the green to yellow part.
Examples of fluorescence
Certain versions of the Pantone guide had a few cards with the ever-popular 800 series inks. These inks all have fluorescent properties.
One of the more hip of these colors is Pantone 804, which is the orange ink. I almost called this dayglo orange, but that would be a misuse of the word, since Dayglo is a company. They make Dayglo pigments.
To demonstrate the phenomenal fluorescent properties of Pantone 804, I set up my spectrometer, my camera, and dug out my red, green, and blue laser pointers. (Note the repetitive use of the first-person pronoun my. It's all about me. Even when it's not, it's still about me.)
Here is what happens when I point the green laser pointer at Pantone 804. There is a strong peak in the green, at 546 nm. This is the reflection of the light from the laser pointer. But note the broader spectral stuff that appears from 560 nm to 700 nm. Lasers only put out a very narrow range of wavelengths. The broader peak must be fluorescence.
Remember back when I took note of the little bump in the spectrum when I used the blue laser pointer? You may have guessed by now. It was stilbene. The paper that the Pantone book is printed on has quite a bit of FWAs. It's kinda hard to find paper today that doesn't.
Well. Look at the time! It's about time to wrap up this blog post on the nature of emitted light. Today I taught you everything I know (and a little bit more) about things that fluoresce in the night. There was something else I wanted to say about fluorescent light... Can't remember what it was. I guess it can wait until the next blog post. That one will be about fluorescent bulbs. I promise.
The distinction between fluorescence and phosphorescence is thus strictly anthropocentric. Just like the distinction between electromagnetic radiation and light (described in a previous blog post), the distinction is along a continuum and is not based on anything physical other than our meager, pitiful senses.
Examples of phosphorescence
Back in the olden days, engineers made a lot of use of phosphorescence. Cathode ray tubes (CRTs) in electron microscopes and in radar systems had long-persistence phosphors so that the image stayed latent on the tube long enough for us to notice. Quick show of hands... how many in the audience have used one of these devices?
A vintage scanning electron microscope (left) and a vintage radar tube (right)
Ok... let's try to open this up a bit. Show of hands again. How many in the audience have been to a historical museum (or my basement, same thing) and saw a TV that was two feett deep and weighed more than a pregnant and cross-eyed mule? That, my friend, was a cathode ray tube display, with a phosphor on the inside of the display end of the tube. Then again... maybe that was more accurately called a fluorescor, since we definitely didn't want it to persist for more than a 30th of a second.
(By the way, a scanning electron microscope is nearly identical in structure to the cathode ray tube in a television set. In fact, the cathode ray tube display was invented along side the scanning electron microscope. Someday I will blog on that topic.)
Certain lichens and mushrooms will glow in the dark long after the sun has gone down. Perhaps there is an evolutionary advantage to being seen as a part of the night life of the forest? I dunno. When it comes down to it, being a visible part of the night life has never given me much of an evolutionary advantage. It usually kicks my wife up into a higher energy state.
Some minerals fluoresce like an Anglo-Swedish color scientist with sunburn. Party-loving minerals like fluorite come to mind. I wonder where it got that cool name? Come to think of it, where did phosphorous get it's cool name?
Welcome back to the 60's
You can also see phosphorescence if you look at a fluorescent bulb in the dark, just after it has been turned off. Here we see the vague distinction between fluorescent and phosphorescent. Some of the stuff inside the tube is fluorescing, and some of it is phosphorescing. But more on that when I finally get around to discussing fluorescent bulbs.
But by far the most useful application is the little rubber duckie that was sitting on my wife's desk, at least until I absconded with it for a photo shoot. The rubber duckie is impreganted with some phosphor with excitation in the violet to blue part of the rainbow, and emission in the green to yellow part.
You can't claim to be uber-cool until you have one of thes on your desk
Examples of fluorescence
Certain versions of the Pantone guide had a few cards with the ever-popular 800 series inks. These inks all have fluorescent properties.
Picture of my 2005 Pantone guide
One of the more hip of these colors is Pantone 804, which is the orange ink. I almost called this dayglo orange, but that would be a misuse of the word, since Dayglo is a company. They make Dayglo pigments.
To demonstrate the phenomenal fluorescent properties of Pantone 804, I set up my spectrometer, my camera, and dug out my red, green, and blue laser pointers. (Note the repetitive use of the first-person pronoun my. It's all about me. Even when it's not, it's still about me.)
Here is what happens when I point the green laser pointer at Pantone 804. There is a strong peak in the green, at 546 nm. This is the reflection of the light from the laser pointer. But note the broader spectral stuff that appears from 560 nm to 700 nm. Lasers only put out a very narrow range of wavelengths. The broader peak must be fluorescence.
Now have a look at the spectrum emitted when the blue laser pointer is swapped in. The laser wavelength appears way far to the left, tucked away nicely at 390 nm. Then there's a broad peak that looks a lot like the broad peak in the previous spectrum. The excitation wavelength has changed, but the emission spectrum has not.
Or at least the fluorescent emission spectrum hasn't changed a lot. Have a close look at the region from 460 to 510 nm. We see another bump. Not a big one, but a bump all the same. Why didn't this show up in the experiment with the green laser?
The explanation can be found above. I don't mean in the Heavens, but earlier in this blog post. I wisely said: "each fluorescent emission is at a lower energy than the excitation." The green laser just didn't have the gumption to excite emission in the blue part of the spectrum.
This should help us explain the frankly quite boring results with the red laser pointer that are shown below. We see a red peak, which is way up at 688 nm. Ho-hum. Any fluorescence would have to be above that, so we get bupkis in the way of fluorescence.
Fluorescent whitening agents
How about another example? I have previously touched on the topic of additives that make paper whiter. I mentioned it in a blog about color-related standards in the print industry. I also blogged about how different spectros deal with the problems caused by the whitening stuff. And I blogged about a conference with a sub-conference on the little buggers.
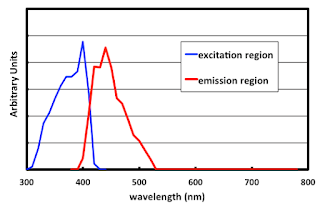
The image below shows the reflectance spectrum of one paper stock. You might notice something a bit peculiar about it, especially around 430 nm. Go ahead. Have a look. And take note of the scale on the left-hand side. The observant reader will have noticed that over 120% of the light that hits the surface is reflected back. For the mathophobes in the crowd, 120% is more than 100%. So... this paper is creating light?
Note the attractive little bump at the blue end of the spectrum
So, here's the scam. Paper normally looks like a brown paper bag. You can make it whiter by various means, including bleaching it, but that's expensive. Not horribly expensive, but there is cost involved. And people like their paper to be white. In fact, studies have shown that people prefer paper that is just a tad on the blue side of true white.
A cheaper way to get white (and the only way to get blue) is to add fluorescent whitening agents to the paper. There is a family of compounds known under the name of stilbenes. Below is the excitation / emission spectra of stilbene stolen from a TAGA paper by Dr. David Wyble and some Anglo-Swedish guy who likes to think of himself as a color scientist. The blue line shows the amount of energy that the stilbene absorbs, as a function of wavelength. Note that this is in the UV region, mostly all between 300 nm and 400 nm. The red line is the wavelengths where that energy is fluorescently emitted. Pretty much what we would call the blue region of the spectrum, from 400 nm to 500 nm.
Yest'day I's fluorescin', and today, I still-been fluorescin'
Adding stilbene to a paper stock will boost the blue. Since drab, dull, yellowish paper is blue-deficient, this will make it look whiter. Well, provided there is some UV light to get it excited. Paper is not creating light, it's redistributing the energy from the UV to higher wavelengths.
The image below illustrates that. There are three sheets of paper here. I wrote on them, annotating the amount of FWAs. On the right side, I took a picture of the three sheets under regular old garden-variety light. The three look similar. On the left we have a picture of those same three sheets under a UV flashlight. OMG! It is pretty obvious that there is some sorta difference going on!
Three sheets to the fluorescent wind
BTW, FWA AKA OBA. Someone got the bright idea to call these brighteners OBAs. This stands for Optical Brightening Agents. I agree, the term fits. Stilbene brightens paper optically. But so does bleach, calcium carbonate, and titanium dioxide, and a good coat of white paint. These four will all increase the reflectance of paper in the blue region. But only stilbene does it with a fluorescent flair. So, if you hear someone call stilbene an OBA, wag your finger at them and tell 'em John the Math Guy says that they are using the term improperly.
Remember back when I took note of the little bump in the spectrum when I used the blue laser pointer? You may have guessed by now. It was stilbene. The paper that the Pantone book is printed on has quite a bit of FWAs. It's kinda hard to find paper today that doesn't.
Well. Look at the time! It's about time to wrap up this blog post on the nature of emitted light. Today I taught you everything I know (and a little bit more) about things that fluoresce in the night. There was something else I wanted to say about fluorescent light... Can't remember what it was. I guess it can wait until the next blog post. That one will be about fluorescent bulbs. I promise.